The food a child eats plays a key role in their health throughout both their infant and adult lives. Nothing can ever quite replace the quality of breast milk, and offering baby formulas with a nutritional profile suited to new-borns’ needs is a real challenge for nutrition experts. In this article, we look at why baby formula needs to be able to adapt to a new-born’s ever-changing needs, and how innovation and the decision to use specific ingredients impact on the quality of the finished product.
SPOTLIGHT ON THE NUTRITIONAL NEEDS OF NEW-BORNS AND CHILDREN
Defining infant nutrition
Infant nutrition is one of the most decisive factors in a child's growth and development. Paediatricians believe that the nutrition provided in a child's first 1,000 days, from conception to their 24-month birthday, is crucial not only to how the new-born grows but the health they can expect to go on to enjoy as an adult. The WHO recommends feeding an infant breast milk exclusively during the first six months of their life. Breast milk boosts natural defences, stimulates growth and naturally adapts to the child's changing nutritional needs.
The right nutrition at the right age
- In some circumstances, a mother may need to substitute breast milk with “infant formula/first formula” or "baby formula" which can be used exclusively up to six months.
- From six to 12 months, babies can be given “second formula” or “follow-on formula".
- Alongside these formulas, suitable food in terms of nutrition as well as in terms of taste and texture can be introduced, such as dairy products, compotes and jar food. Food must have the right nutritional profile, but it must also have the right sensory aspects to help develop the child's palate.
- “Growing-up milk” can be used alongside second formula for young children aged one to three.
Children's nutritional needs evolve as they grow. In the first year of a child's life, a new-born grows extremely quickly, gaining around 25 cm (50% of their total height) and almost six kilos, which is why the recommended daily intake and nutritional values are so specific and change so rapidly over their first few years.
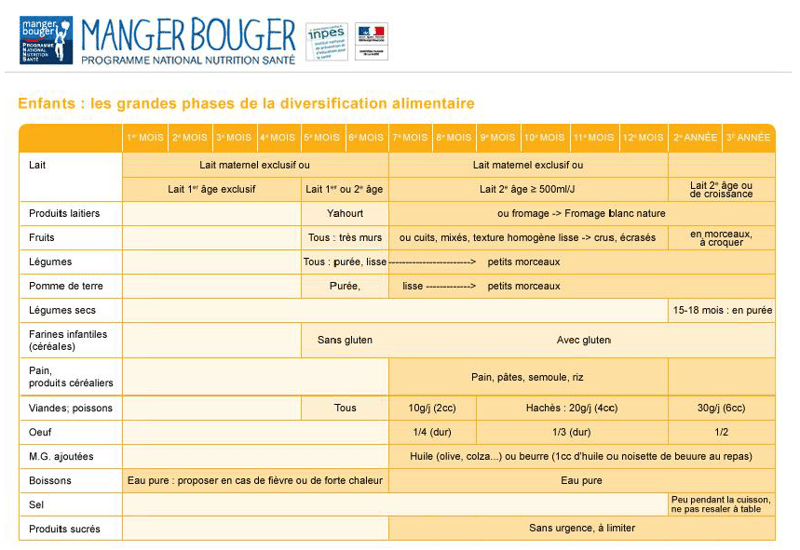
Source: Programme National Nutrition Santé (France)_INPES
Suitable, regulated food products
Baby food products are governed by stringent regulations to ensure food for new-borns is safe and of the highest possible quality. Manufacturers must remain vigilant and aware of key points to consider before launching infant formula and products on a new market.
Looking for more information on infant food regulations? A couple of useful references (non-exhaustive): EU DIRECTIVE 2006/141, ACTE DÉLÉGUÉ 2016/127, CODEX STAN 72-1981 rev. 2007, GB 10765-2010 and GB 10767-2010.
THE RIGHT INGREDIENT FOR THE RIGHT INFANT FORMULA
Product innovation put to use for unprecedented health benefits
According to Innova Market Insights, despite severe regulatory constraints, infant formula specialists still have room to develop their range of products in other applications and develop real added nutritional benefits for new-borns including:
- Premature babies
- Treating and preventing allergies
- Cognitive development
- Gastro-intestinal health and digestive comfort
- Natural defences and immunity
- And even preventing adult obesity with low-protein formulas
New-borns are fragile and frequently have digestive and immune system imbalances. Ingredients are now available to develop high nutritional value infant formulas that meet babies' needs.
Here are a few examples of new-borns’ specific needs and their solutions:
#1 Ingredients to promote new-born digestion

Baby food manufacturers can, in some circumstances, develop nutritional solutions that impact on new-borns’ digestive health and comfort. These benefits are particularly visible when probiotics, prebiotic fibres, lactoferrin and hydrolysed proteins are used in infant formulas. Some children are very sensitive to lactose and can experience diarrhoea as a result. In these cases, new-borns who are not breastfed should be fed a low-lactose infant formula. This is why we always offer customers potassium caseinate and calcium, which are specifically recommended for use in low-lactose formulas.
#2 Bio-available minerals to boost growth
The EFSA scientific group concluded that a daily calcium intake of 200 mg in the first semester and 400 mg per day in the second half of a child's first year were sufficient for most new-borns. But it's not all about the quantity! Quality and bio-availability are key criteria to consider. Formula manufacturers must focus on balancing calcium and phosphate levels, which ensures the organism can absorb the minerals in question. In terms of weight, the calcium/phosphate ratio of breast milk sits around 2:1.
#3 High-quality protein

According to the EFSA's scientific report, all infant formulas must be safe and adapted to new-borns’ needs while promoting growth and development. The EFSA recommends a minimum protein intake of 1.8 g/100 kcal of infant formula, sourced from cow or goat milk proteins.
To meet the regulatory level of aminograms, ARMOR PROTEINES offers a range of highly purified milk protein ingredients. Introducing LACTARMOR DM 90, POTASSIUM CASEINATE AND CALCIUM by ARMOR PROTEINES.
#4 Active ingredients for total protection
Research shows that some ingredients could have huge functional value for their physiological impact on new-borns’ overall health. Lactoferrin, for example, is believed to contribute to infection resistance and digestive system maturation in new-borns, and particularly in premature babies.
In addition, it is believed to boost cognitive function during the rapid brain growth period.
Example of maximum 'safe' doses for lactoferrin in infant formulas: 1) The EU’s new Novel Food regulation: 100 mg/100 ml of reconstituted milk. 2) GRAS USA: 100 mg/100 g of powder infant formula.
A RANGE OF INGREDIENTS PERFECTLY SUITED TO INFANT FORMULAS
ARMOR PROTEINES offers a range of ingredients that meet the highest of safety requirements and regulations. Formula manufacturers are given access to a wide range of essential amino acids and proteins, minerals and other active ingredients that contribute to a child's optimum growth from pregnancy to after birth.
A market leader in sourcing dairy ingredients for infant formulas, ARMOR PROTEINES offers manufacturers tailored support from design to formulation.
Sources :
- Scientific Opinion on the essential composition of infant and follow-on formulae1, EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), 2014.
- http://inpes.santepubliquefrance.fr/CFESBases/catalogue/pdf/890.pdf
- Vidailhet M. et Turck D. Besoins nutritionnels : définition des apports nutritionnels conseillés. In : O Goulet, M Vidailhet, D Turck, coordinateurs. Alimentation de l'enfant en situation normale et pathologique. 2ème édition, Doin éditeurs, Paris. 2012 : 59-70
- American academy of pediatrics. Caring for your baby and young child: birth to age 5, Bantam Books. 2004. 752 pages
- Legrand D, Overview of Lactoferrin as a Natural Immune Modulator J Pediatr. 2016 Jun;173 Suppl:S10-5.
- Euromonitor International (2015) Market Overview: Identifying New Trends and Opportunities in the Global Infant Formula Market, Part I. (Aperçu du marché : Identification des nouvelles tendances et opportunités sur le marché mondial des laits infantiles. 1e partie.) [EN LIGNE] Disponible (en anglais) à l’adresse suivante.
- WHO recommendations on maternal health guidelines approved by the who guidelines review committee updated may 2017
- Organisation mondiale de la santé (2017): Alimentation du nourrisson et du jeune enfant: Aide-mémoire. [EN LIGNE] Disponible à l’adresse suivante.
- Scientific Opinion on the essential composition of infant and follow-on formulae, EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), 2014
- Legrand D, Overview of Lactoferrin as a Natural Immune Modulator J Pediatr. 2016 Jun;173 Suppl:S10-5
 Emilie SCOTTI
Emilie SCOTTI
-3.jpg)




